

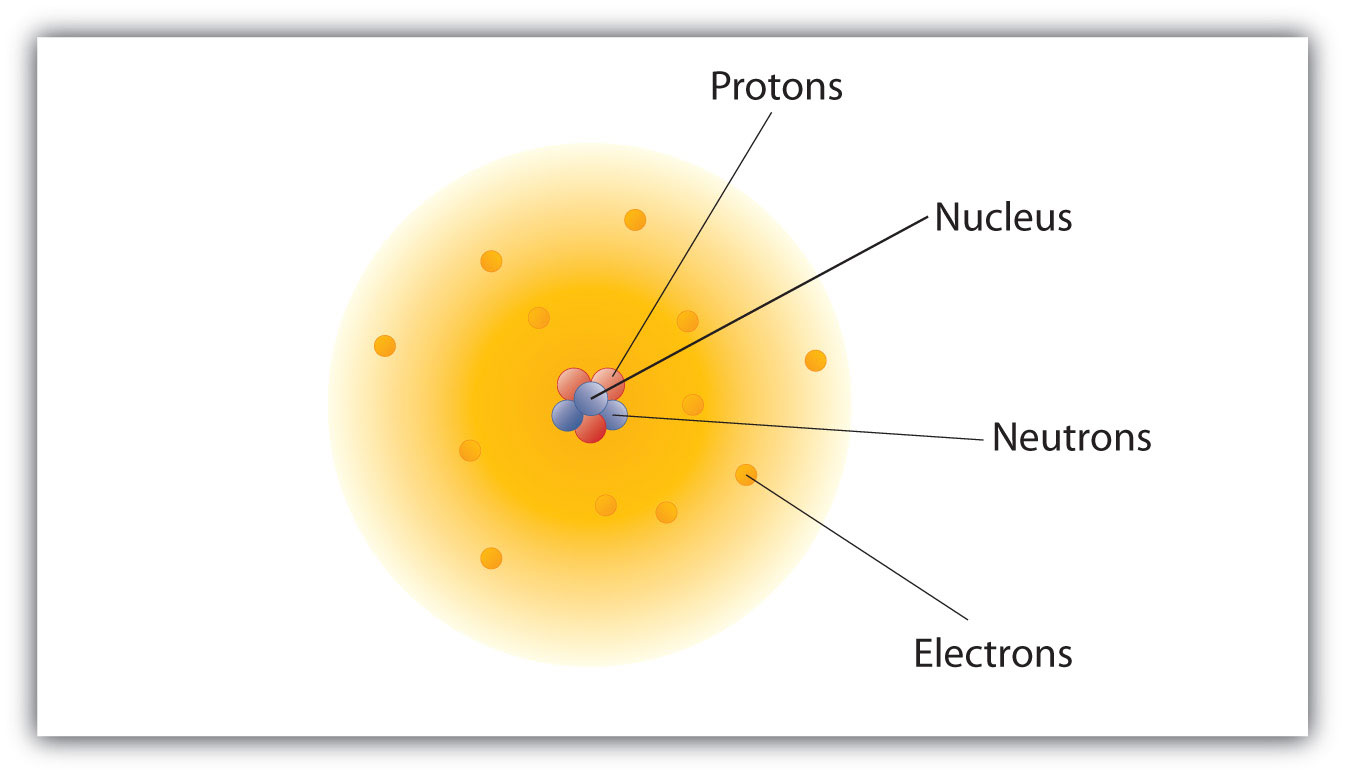
The video at the URL below is a good introduction to modern atomic theory. Bohr’s model was an important step in the development of modern atomic theory. He elaborated a system originated by his teacher Leucippus into a materialist. Democritus, known in antiquity as the ‘laughing philosopher’ because of his emphasis on the value of ‘cheerfulness,’ was one of the two founders of ancient atomist theory. The Law of Definite Proportions applies when elements are reacted together to form the same product. Danish physicist Niels Bohr created a more accurate and useful model. First published Sun substantive revision Sat Jan 7, 2023. These atoms consist of subatomic particles: neutrons, electrons. Law of Definite Proportions states that in a given type of chemical substance, the elements are always combined in the same proportions by mass. All the matters composed of small particles known as atoms. This page contains materials for the session on the atomic models of Rutherford and Bohr. Similarly, when 2 grams of A react with 16 grams of B, they must produce 18 grams of C.
#Modern atomic model full
If 1 gram of A reacts with 8 grams of B, then by the Law of Conservation of Mass, they must produce 9 grams of C. Niels Bohr, in full Niels Henrik David Bohr, (born October 7, 1885, Copenhagen, Denmarkdied November 18, 1962, Copenhagen), Danish physicist who is generally regarded as one of the foremost physicists of the 20th century.
\): If 1 gram of A reacts with 8 grams of B, then by the Law of Definite Proportions, 2 grams of A must react with 16 grams of B.


 0 kommentar(er)
0 kommentar(er)
